💯 What You Need To Do Before Classes Start:
💼 Jobs & Internships
Internship season is in full swing! The race is on!
Freshmen - Check out our 4-Year Plan for how to get internships at startups!
Sophomores - Apply To These Positions
General Mills R&D Intern
L3 Harris Mechanical Engineering Intern
Juniors - Full Send Longshots!
Seniors & Graduates - It’s Go (Full) Time!
Super Tough:
More Reasonable:
Not seeing what you are looking for? Check out our Job Board for more MechE positions!
🖥️ Onshape Tip
In the last 3 months I have finally become a hole feature user after 9 years of sketching holes.
Why . . . ? Well I just can’t trust NX sketches to update (some might say a “skill issue”)
Onshape takes the simple hole tool and makes it your best friend . . . why? . . . because it checks your work for you.
In this very very real part below. I have 5x hole operations with 4x different hole sizes.
With using the hole feature in any CAD software the problem I always run into is that the features disappear in the massive feature tree. When I change a hole from 8.75mm to 8.6mm to get my M6 PEM nuts not to spin on me I have to make sure I update every single instance of 8.75 holes.
Onshape’s hole table consolidates this for you and allows you to see if there are any 8.75mm holes that haven’t been updated!
👶 Meme Of The Week
🙋♂️ Interview Practice Question of the Week | Tesla
You are boiling some water in the breakroom for afternoon tea when your boss walks in. He fills a coffee cup with some water from the sink and sets it in your pot of boiling water before asking you one simple question:
Will the water in the coffee cup boil?
✅ The Answer
There’s a surprising amount to think about here for a question so simple.
To be honest, when I first heard this question, my instinct was to say yes (with only limited reasoning why). The water in the pot is boiling and will eventually heat the coffee cup and the water to boiling temperature via convection. This is true more or less. So what would the temperature curve look like of the coffee cup?
If you’re familiar with Newton’s law of heating/cooling, you should know that the heat flux is proportional to the difference in temperature. In this case, the difference in temperature starts out large, but as the coffee cup temperature rises, the boiling water temperature stays the same (due to heat input from the stove). The delta therefore goes down as does the heat flux. The coffee cup temperature will thus asymptotically approach boiling temperature. At some point, it can be considered to be at effectively 100 C.
Note that an interviewer may choose to dive in here and ask you more about how long it may take the coffee cup to reach 100 C, especially if it’s for a heavily thermal based role. Discussion about heat transfer via turbulent vs laminar convection, how to use and meaning of the biot number, and “lumped capacitance” first order solutions are all on the table (IMO that would be a brutal tangent for the interviewer to go on, and is very unlikely unless the applicant has a strong background in heat transfer or the role is heavily thermal analysis based). More likely would be a request to draw a thermal resistance diagram of the system.
The coffee cup (and the water in the coffee cup eventually, rate mainly dependent on how insulating the cup is) asymptotically approaches the boiling temperature of water. Even at this point, it may seem like it will boil in such a case for practical application. Even though the rate of growth in temperature exponentially decays as it approaches the boiling temperature, it should eventually get “close enough,” right?
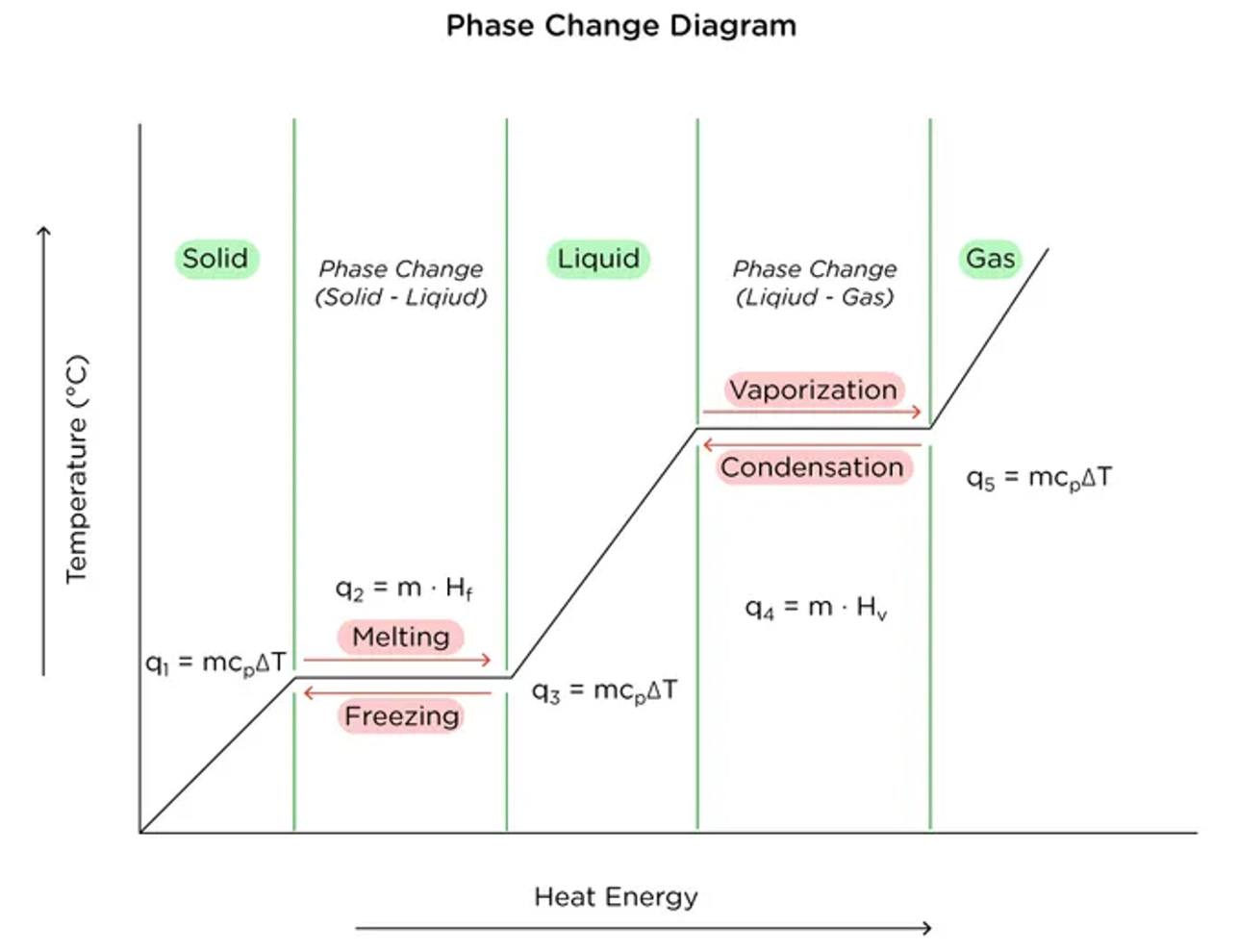
Turns out, that question is the wrong one to ask. Let’s take a look at a standard heating diagram for water to find out why.
To bring water from, let’s say 50 C to a boil (i.e. the gaseous state), it requires energy to raise the temperature (Q=MCdT) and energy to actually vaporize the liquid (Q = ML). The second energy input is typically known as the latent heat of vaporization. During the vaporization process, temperature stays constant. This property is very helpful in cooking and helps us to not burn foods or overheat our pans when enough water is present.
The key here, is that the once the temperatures are the same (at 100 C), the boiling water is incapable of imparting any more energy onto the coffee cup. The water in the pot boils due to an input of heat at the bottom of the pot due to the stove flame directly beneath it. The water at the bottom of the pot vaporizes and bubbles up. Crucially, once it is vaporizes and starts to rise, its temperature cannot rise higher than 100C as it is surrounded on all sides by boiling water (< 100 C). Thus no additional heat energy can be transferred from the boiling pot to the coffee cup. As such, the coffee cup will never gain sufficient heat energy in the current setup in order to overcome the latent heat of vaporization and truly boil!
A great follow up question would be, how can we alter the setup to cause the coffee cup to boil? Here are some options:
Fill the surrounding pot with oil instead of water. Oil boils at a higher temperature, so the coffee cup should get sufficiently hot to boil water. Note that I am not responsible for any and all grease fires due to implementation of this method.
Water actually requires an initial nucleation site to initiate boiling. This is the reason why care is recommended when boiling water in the microwave. In a smooth and clean ceramic cup, it’s possible for the surface to be so smooth that no nucleation site forms. The microwave radiation continues warming up the water past 100 C. When disturbed (usually by someone taking their hot water out of the microwave), the agitation spawns a nucleation site and the cup of water erupts! Boiling over and burning the bewildered holder. We can use potentially use this effect to our advantage. By minimizing nucleation sites (might also require a more uniform heating of the system than a bottom stove), the water could be superheated. By providing nucleation inside of the coffee cup, the water inside the cup will boil (ironically in this situation, the water in the cup would boil but the water outside the cup may not).
After boiling the water for some time (coffee cup reaches thermal equilibrium at 100 C), put on a lid and pump out the air from the coffee pot / cup. The decrease in pressure will reduce the boiling point drastically and the water in both the pot and the cup should boil.
More realistically, this might be able to be accomplished using a sealable lid that has been in the freezer. When it seals to the pot, it will cool any water vapor floating up and making contact, causing the vapor to condense. Given water vapor is ~1000x less dense than water, this should result in a substantial drop in pressure. That drop in pressure may be enough to cause the water inside the pot to momentarily boil (releasing more vapor and ruining the vacuum until the vapor recondenses). Note that I have no idea if this idea would work in reality as it is purely based on conjecture.
My personal favorite: boil off the water in the pot. Eventually the coffee cup will reach the bottom, at which point conduction will not be limited by the boiling temperature of water and the stove’s heat will get transferred to the cup, boiling the water in the cup.
Pressure cookers work based off of a similar principle. Instead of creating a vacuum, they add pressure to the system (usually by containing the steam from boiled water). With higher external pressure, the boiling temperature goes up, allowing food inside (like rice) to cook faster and at higher temperature.
Regardless, in summary, the coffee cup will not boil without intervention! Keeping a level head and thinking through the fundamentals should always be able to get you to the answer, even if it takes a few minutes. This one is a great question because it rewards depth of thought and knowledge of first principles over conjecture while simultaneously giving space for quantitative estimation or creative solutions depending on inclination. Hope you learned something!